Introduction:
Acquired von Willebrand syndrome (AvWS) has been associated with Philadelphia-negative chronic myeloproliferative neoplasms (MPN) since 1984. However, the epidemiology of these disorders is not precise and has been underestimated worldwide but prevalence of 10-20% has been described in other series. Determinations of the antigenic von Willebrand factor (VWF:Ag) and its activity (VWF:RCo) are only performed when patients present thrombocytosis over 1,000 x10 3/µL. Essential Thrombocythemia (ET) is the most commonly associated MPN, found in 5-30%. Some cases have been described in patients with polycythemia vera (PV) and primary myelofibrosis (PMF) who develop AvWS even with platelet counts below 1,000 x10 3/µL. The epidemiology of AvWS in MPN and its association between laboratory and molecular parameters among the Mexican population have not been described.
Objectives
- Describe the clinical, biochemical and molecular characteristics of patients who develop AvWS with the aim of establishing the difference between cut-off scores of platelets to suspect AvWS in Mexican patients.
- Establish the association between mutations in JAK2, CALR and MPL, and the appearance of AvWS.
Material and methods:
Retrospective, descriptive, observational, and single-center analysis.
Patients over 18 years of age with a diagnosis of MPN from the INCMNSZ were recruited. The mutational analysis in peripheral blood (PB) for JAK2, CALR and MPL was assessed, with at least one follow-up between 2012 and 2022, excluded whose medical record were incomplete.
Information was consecutively collected from 184 patients with MPN diagnosis between 01/01/2000 to 05/31/2022. Thirty patients were identified as having AvWS with a VWF:RCo/VWF:Ag diagnostic ratio of < 0.7. The epidemiology, clinical, laboratory and mutational alterations of the patients who developed AvWS by was analyzed using descriptive statistics with SPSS. This study was approved by the institutional bioethics committee.
Results:
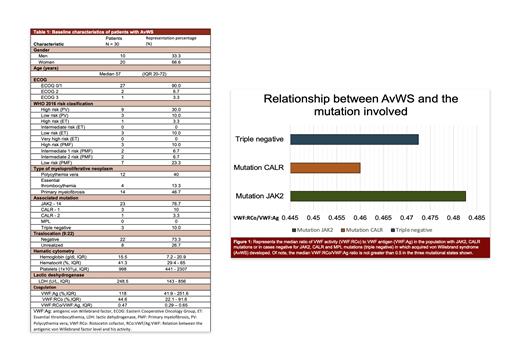
AvWS developed in 16.4% (n = 30) of patients with MPN with an annual incidence of 2.4 cases per year was identified. Most patients were women in 66% (n = 20), with a median age of 57 years (IQR 20-72 years). Of the thirty cases with AvWS, 46.7% (n =14) corresponded to PMF, 40% (n =12) to PV, and 13.3% to ET (n = 4). The most frequently found mutation was JAK2 exon 14 with 76.7%, followed by CALR in 13.3% and triple negatives in 10%, no patient presented MPL mutations. The global median of VWF:RCo/VWF:Ag was 0.47 [PV 0.47 (IQR 0.31-0.64), ET 0.53 (IQR 0.5-0.64) and PMF 0.48 (0.29-0.65)] (Figure 1) and the median of the VWF:Ag 118 U/dL with FVW:RCo of 44.6 UI/dL (Table 1). The median platelet count for patients with the JAK2 mutation was 987.9 x103/µL (IQR 218 - 2307 x10 3/µL), while those with CALR and triple negatives were 1,033 x10 3/µL (IQR 713 - 1339 x10 3/µL), and 1,325 x10 3/µL (IQR 760 - 2059 x10 3/µL), respectively. Regarding hemoglobin (Hb) and hematocrit (Hto) levels, patients with PV and JAK2 mutation with AvWS presented medians of 17.45 g/d and 54.4% respectively, highlighting that AvWS was generated in some patients with platelet counts below 1,000 x10 3/µL. At diagnosis, bleeding episodes occurred in 13.3%. Lastly, 96.7% of the patients were treated with hydroxyurea, which corrected cellular alterations and laboratory abnormalities found in AvWS.
Conclusions:
AvWS can be found in patients with platelet counts below 1,000 x10 3/µL, especially among those with PV with elevated Hb and Hct levels. This may deem it necessary to carry out diagnostic tests in patients with MPN and any degree of thrombocytosis. To our knowledge, this is the most comprehensive study among latin american patients that describes the coexistence of PMF and AvWS; even though the mechanism by which some PMF patients develop AvWS is not perfectly understood. In our study, patients with PMF presented AvWS more frequently; this is a contrasting result from other international reports where ET was most attributed. We attribute this finding to changes made to the WHO criteria between 2008 and 2016.
Disclosures
Apodaca Chavez:Astra Zeneca: Speakers Bureau. Tuna Aguilar:MSD: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Abbvie: Speakers Bureau; Pfizer: Speakers Bureau.